Experiment No. 5 Limit test for Arsenic
Limit test for Arsenic
Principle:
Limit test of Arsenic is based on the reaction of arsenic gas with hydrogen ion to form yellow stain on mercuric chloride paper in presence of reducing agents like potassium iodide. It is also called as Gutzeit test and requires special apparatus.
Arsenic, present as arsenic acid in the sample is reduced to arsenious acid by reducing agents like potassium iodide, stannous acid, zinc, hydrochloric acid, etc. Arsenious acid is further reduced to arsine (gas) by hydrogen and reacts with mercuric chloride paper to give a yellow stain.
H3AsO4 + H2SnO2 → H3AsO3 + H2SnO3
Arsenic acid Arsenious acid
H3AsO3 + 3H2 → AsH3 + 3H2O
Arsenious acid Arsine
The depth of yellow stain on mercuric chloride paper will depend upon the quality of arsenic present in the sample.
Procedure:
Test solution:
The test solution is prepared by dissolving specific amount in water and stannated HCl (arsenic free) and kept in a wide mouthed bottle.
To this solution 1 gm of KI, 5 ml of stannous chloride acid solution and 10 gm of zinc is added (all this reagents must be arsenic free)
Keep the solution aside for 40 min and stain obtained on mercuric chloride paper is compared with standard solution.
Standard solution:
A known quantity of dilute arsenic solution is kept in wide mouthed bottle and rest procedure is followed as described in test solution.
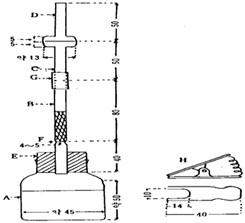
A : approximately 60 ml generator bottle with 40 ml indicating line.
B : glass tube with 6.5 mm inner diameter
C and D : a ground joint glass tube with 6.5 mm inner diameter and 18 mm outer diameter at the joint. Inner joint and the outer joint form a concentric circle.
E : rubber stopper
F : narrow part of the glass tube B. Glass wool is inserted up to this part.
G : rubber board (Lead acetate cotton plug)
H : clamp
Reasons:
Stannous chloride is used for complete evolution of arsine
Zinc, potassium iodide and stannous chloride is used as a reducing aget
Hydrochlorid acid is used to make the solution acidic
Lead acetate pledger or papers are used to trap any hydrogen sulphide which may be evolved along with arsine.
Limit test of Arsenic is based on the reaction of arsenic gas with hydrogen ion to form yellow stain on mercuric chloride paper in presence of reducing agents like potassium iodide. It is also called as Gutzeit test and requires special apparatus.
Arsenic, present as arsenic acid in the sample is reduced to arsenious acid by reducing agents like potassium iodide, stannous acid, zinc, hydrochloric acid, etc. Arsenious acid is further reduced to arsine (gas) by hydrogen and reacts with mercuric chloride paper to give a yellow stain.
H3AsO4 + H2SnO2 → H3AsO3 + H2SnO3
Arsenic acid Arsenious acid
H3AsO3 + 3H2 → AsH3 + 3H2O
Arsenious acid Arsine
The depth of yellow stain on mercuric chloride paper will depend upon the quality of arsenic present in the sample.
Procedure:
Test solution:
The test solution is prepared by dissolving specific amount in water and stannated HCl (arsenic free) and kept in a wide mouthed bottle.
To this solution 1 gm of KI, 5 ml of stannous chloride acid solution and 10 gm of zinc is added (all this reagents must be arsenic free)
Keep the solution aside for 40 min and stain obtained on mercuric chloride paper is compared with standard solution.
Standard solution:
A known quantity of dilute arsenic solution is kept in wide mouthed bottle and rest procedure is followed as described in test solution.
A : approximately 60 ml generator bottle with 40 ml indicating line.
B : glass tube with 6.5 mm inner diameter
C and D : a ground joint glass tube with 6.5 mm inner diameter and 18 mm outer diameter at the joint. Inner joint and the outer joint form a concentric circle.
E : rubber stopper
F : narrow part of the glass tube B. Glass wool is inserted up to this part.
G : rubber board (Lead acetate cotton plug)
H : clamp
Reasons:
Stannous chloride is used for complete evolution of arsine
Zinc, potassium iodide and stannous chloride is used as a reducing aget
Hydrochlorid acid is used to make the solution acidic
Lead acetate pledger or papers are used to trap any hydrogen sulphide which may be evolved along with arsine.
Comments
Post a Comment